Translate this page into:
Understanding the essentials of photodynamic therapy for dental practitioners: Shining a spotlight
*Corresponding author: Prateek Srivastava, Department of Oral Medicine and Radiology, Saraswati Dental College and Hospital, Lucknow, Uttar Pradesh, India. prateeksri13@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Srivastava P, Sinha S, Chandra S, Singh SK. Understanding the essentials of photodynamic therapy for dental practitioners: Shining a spotlight. Asian J Oral Health Allied Sci. 2024;14:12. doi: 10.25259/AJOHAS_8_2024
Abstract
Background:
Oral disorders, including tooth decay, periodontal disease, and endodontic infections, are prevalent worldwide. The escalating issue of antibiotic resistance exacerbates the need for alternative treatment modalities.
Aim:
This review aims to provide a comprehensive overview of advancements in photodynamic therapy (PDT) for dental applications, highlighting its potential as a non-invasive local and adjuvant therapy for various oral infectious disorders. PDT, which originated in the early 20th century, involves three primary components: A photosensitizer (PS), light, and oxygen. The PS selectively targets unwanted eukaryotic cells, such as tumors in the oral cavity, or pathogenic microorganisms, while sparing healthy cells. The efficacy of PDT is significantly influenced by the type of PS utilized.
Method:
An electronic search was conducted using the PubMed database focusing on peer-reviewed articles up to November 2023 using the keywords “photodynamic therapy,” “photochemotherapy,” “antimicrobial photodynamic therapy,” “antimicrobial photodynamic therapy oral,” resulting in 2341, 1034, 389, 221, and 96 items, respectively. After appropriate review, relevant data from other electronic sources are also included for additional information.
Results:
Historical and recent studies have demonstrated the effectiveness of porphyrin PSs and temoporfin in treating premalignant and malignant intraoral lesions, particularly as palliative treatments when conventional therapies have failed. In addition, phenothiazinium PSs, such as methylene blue and toluidine blue, have shown substantial antimicrobial activity against bacteria, viruses, and fungi. PDT has been applied successfully to treat specific oral conditions, including caries, endodontic and mucosal infections, periodontal disorders, and periimplantitis. Notably, its antibacterial properties suggest that PDT could reduce the dependency on systemic antibiotics.
Conclusion:
PDT emerges as a promising alternative to conventional treatments for oral infections, offering a potential solution to the global challenge of antibiotic resistance. Continued research and development are essential to enhance the effectiveness and broaden the application of PDT in dental care.
Keywords
Photodynamic therapy
Antibiotic resistance
Photosensitizers
Dental treatment
Non-invasive therapy
INTRODUCTION
Photodynamic therapy (PDT) as a treatment modality has been developing in various medical specialties since the 1960’s. It can be defined as light-induced photo-inactivation of cells/microorganisms or molecules.[1]
It is also known by a variety of names such as photo-activated disinfection, photoactivated chemotherapy, photo radiation therapy, and phototherapy or photo chemotherapy.
Photodynamic treatment uses photosensitizers (PSs) to selectively eliminate diseased cells by absorbing light at the right wavelength, making it well-tolerated by patients due to its targeted action.
Photodynamic procedures are an outpatient treatment for drug-resistant bacterial infections and chronic inflammation, offering ease of administration and pain-free treatment. They are a non-invasive approach to medicine, used in various medical specialties due to their good treatment outcomes and the ability to be combined with other treatments.[2]
Indian and Egyptian physicians used PDT more than 6000 years ago. Egyptians believed that sunburns could be healed with light-sensitive compounds (psoralens), while Indians thought that extracts from Psoralea corylifolia and light could cure vitiligo. It was discovered around 1250 AD that psoralens were phototoxic.[1]
Niels Finsen, a Danish physicist, developed phototherapy principles using ultraviolet radiation and active rays in the 1890s. His groundbreaking work, which led to the Nobel Prize in 1903, led to the treatment of lupus vulgaris and smallpox.[3] German medical student Oscar Raab discovered that paramecia bacteria, when cultured with acridine orange dye during a rainstorm, perished more quickly, similar to those exposed to sunlight[4] [Figure 1].

- (a) Paramecium – an aquatic organism, (b) acridine orange dye, and (c) Lightning (Courtesy Mr. Ashok Pradhan and Ms. Minati Singha, Source, Times of India, 31st July 2016).
von Tappeiner proposed that light may accelerate chemical-biological reactions due to light’s discovery, stating that oxygen is needed for photosensitization, a phenomenon he called “photodynamiche” in German.[5]
This paper aims to provide dental practitioners with a comprehensive understanding of PDT, highlighting its fundamental principles, clinical applications, and potential benefits in dental practice.
Mechanism of photodynamic therapy
A photoactivable substance that is PS, binds to the target cell and can be activated by light of a suitable wavelength.
The essential components required for the PDT are PS, light, and oxygen[4] [Figure 2].
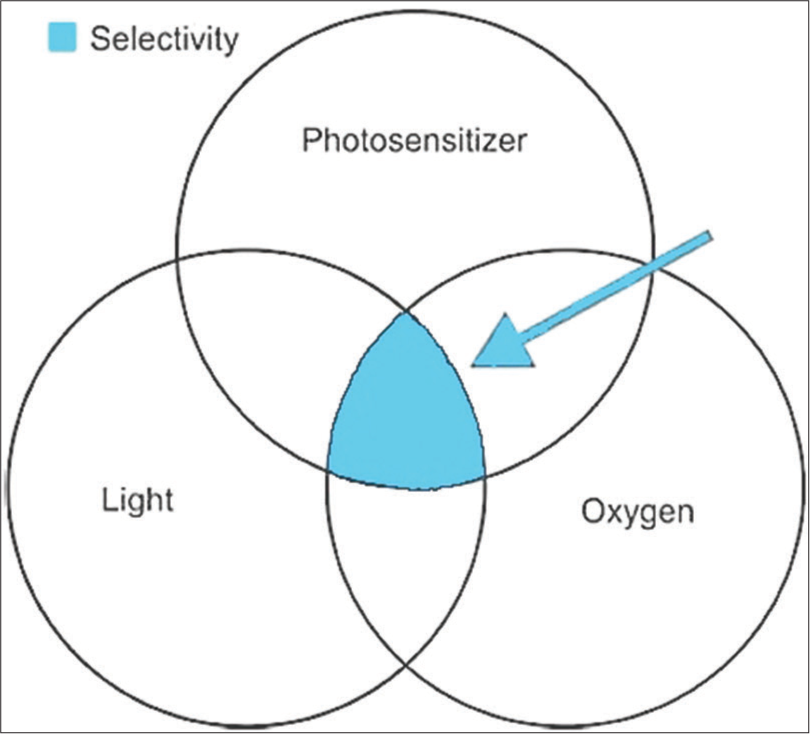
- Venn diagram showing factors for successful photodynamic therapy.
PS agents are unique substances that may be injected, swallowed, or used topically and are utilized in PDT. Commercial availability, affordability, ease of use, long wavelength absorption capacity, significant photocytotoxicity but low dark toxicity, good selectivity toward target cells, and quick elimination are among the best qualities.
They can be classified based on their chemical structure but are more commonly grouped into three broad families based on their clinical characteristics.
First generation
First-generation PSs include substituted derivatives of bacteriochlorin, chlorin, and porphyrin. They are cyclic tetrapyrroles and have been in use since the early 1980s and 1970s. Hemaporphyrin structural derivatives are substances of clinical significance. The following are some of these medications’ drawbacks: high aggregation, poor selectivity, limited solubility, and phototoxicity. Due to this, the majority of PSs are no longer suitable for use in PDT; nonetheless, they have been used as a source for new PSs that adhere to modern pharmaceutical requirements.[6,7]
Second generation
The creation of second-generation PSs started in the late 1980s with the goals of improving pharmacokinetic properties, lowering toxicity, and increasing the efficacy of first-generation medications. Compared to compounds from the first generation, these PSs exhibit near-infrared absorption and strong singlet oxygen (1O2) production. Chlorins, bacteriochlorins, phthalocyanines, macrocyclic compounds, fullerenes, and tetrapyrrolic compounds are a few examples. Tetrapyrrolic compounds and fullerenes, a carbon allotrope structure, are some more novel second-generation PSs.[6,7]
Third-generation PSs are recently created compounds with significant medical applications. They can be conjugated with biological molecules or have inherent “photo-quenching” capabilities, activating at their target location. These compounds can be carrier molecules such as liposomes, polymers, monoclonal antibodies, and polymeric nanoparticles. For the former, they can activate tumor surface markers, receptors, and transporters. The latter group includes tumor surface markers, receptors, and transporters. Polyethylene glycol conjugation of fullerenes improves their solubility in water-based solvents and in vivo biological settings, as well as their tumor localization.[6,7]
The clinical strategy for PDT involves selecting the right light, delivering it to activate the PS, and ensuring an adequate PS concentration in the presence of oxygen at the target tissue. The optimal light spectrum for PDT is between 600 and 900 nm, with wavelengths below 600 nm being inappropriate due to the absorption of most incoming photons by endogenous substances. Photon energy levels beyond 900 nm are insufficient to produce 1O2 species. Most PSs in clinical use are activated by red light, with a wavelength between 630 and 700 nm and a penetration depth range of 0.5 and 1.5 cm. To enable dosage estimates during therapy, the generated light’s intensity should be constant. The earliest PDT light sources were non-coherent lamps, such as metal halide lamps, tungsten filament, quartz halogen, phosphor-coated sodium lamps, and xenon arc lamps. These lamps are safe, affordable, easy to use, and can cover large areas. However, they are not often used due to their high heat, low light intensity, and poor dose control.[6,7]
The photodynamic process in biological systems is oxygen-dependent, requiring the final element oxygen to occur. This oxygen dependence is crucial for the creation of 1O2 and reactive oxygen species (ROS), which are responsible for most photodynamic reactions. Excitation of a PS by a light source initiates the photodynamic process, leading to cell death through photochemical interactions with cellular substrates or molecular oxygen.[6,7]
Classic PDT involves administering a PS to the patient, selectively retaining it in target tissues, and using photochemical reactions, light radiation, and targeted tissue elimination[8] [Figure 3].
![Diagram showing mechanism of photodynamic therapy (type I and type II reactions). PS: photosensitizer, ROS: reactive oxygen species, O2: singlet oxygen, HO: hydroxide, H2O2: hydrogen peroxide, ISC: intersystem crossing, hv: Energy (E=hv). [Adapted from Donohoe et al. 2019].[9]](/content/116/2024/14/1/img/AJOHAS-14-12-g003.png)
- Diagram showing mechanism of photodynamic therapy (type I and type II reactions). PS: photosensitizer, ROS: reactive oxygen species, O2: singlet oxygen, HO: hydroxide, H2O2: hydrogen peroxide, ISC: intersystem crossing, hv: Energy (E=hv). [Adapted from Donohoe et al. 2019].[9]
The PS transitions from an excited singlet state to a low-energy ground state on light exposure, which can either decay back with fluorescent emission or transform into a triplet state, allowing for two different reactions.[7]
Type I: It involves the removal of electrons and hydrogen from a substrate molecule to produce free radicals, generating ions, or directly transferring electrons and hydrogen from the PS. Superoxide, hydroxyl radicals, and hydrogen peroxide are examples of the extremely ROS that are produced when these radicals react quickly with oxygen
Type II: Singlet oxygen, a highly reactive and electrically excited state of oxygen, is produced by the processes.
Types I and II processes both contribute, suggesting that oxygen tension and PS concentration affect the damage mechanism.
Consequently, low doses of PDT-related damage most often lead to apoptosis, whereas high-level damage mainly leads to necrosis.[9]
PDT has been effective against both Gram-positive and Gram-negative bacteria[4] [Figure 4].
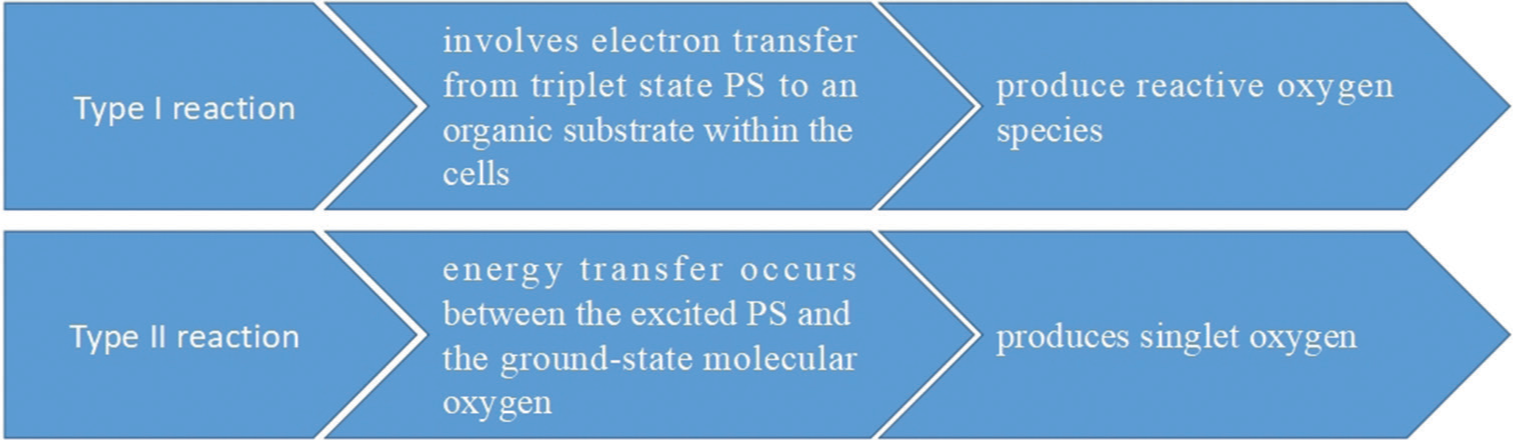
- Mechanism of type I and type II reactions.
Oxidative stress, caused by an imbalance between ROS production and cell repair capacity, leads to various cell death mechanisms. In PDT, apoptosis, autophagy, and necrosis are well-studied forms of death. Necrosis is caused by high photo damage, while moderate damage results in apoptosis and adaptive autophagy. The extent of damage can trigger other controlled cell death pathways[9] [Figure 5].
![Figure showing frequent pathways of apoptosis [Adapted from Donohoe et al. 2019].[9]](/content/116/2024/14/1/img/AJOHAS-14-12-g005.png)
- Figure showing frequent pathways of apoptosis [Adapted from Donohoe et al. 2019].[9]
The treatment enhances healing with minimal intrusive techniques, targeting parasitic protozoa, fungi, yeasts, viruses, and bacteria. It is effective regardless of antibiotic resistance, has no side effects, reduces cancer recurrence likelihood, and is cost-effective to operate. The prevalent clinical applications of PDT in dentistry encompass the treatment of gingivitis, periodontitis, peri-implantitis, halitosis, herpes labialis, caries management, root canal disinfection, and candidiasis.[4,6]
PDT’s efficacy may be compromised in deep tissues with low oxygen levels due to oxygen availability for photochemical reactions. Furthermore, PSs may accumulate under the skin, causing residual skin photosensitivity and potential burns when exposed to daylight. Therefore, direct sunlight exposure must be avoided until medication is completely removed.[4,6]
PDT is a less widely known treatment method, which can lead to delayed or underutilized applications. It often involves specialized equipment, photosensitizing agents, and specific light sources, which can be expensive and limit its widespread adoption. PDT requires meticulous setup, including photosensitizing chemicals and light exposure, which may take time and require collaboration between medical specialists. In addition, logistical difficulties may arise due to controlled lighting requirements in clinical settings. In some cases, the intricacy of the preparation may render PDT impractical, necessitating skilled individuals for its proper implementation.
CONCLUSION
PDT is a promising alternative to traditional medical treatments, offering minimally invasive or non-invasive treatment for various illnesses such as cancer and certain infections. PDT targets aberrant cells selectively while protecting healthy tissues, making it a beneficial choice for patients and healthcare workers. It has shown efficacy in overcoming resistance to conventional treatments, such as chemotherapy, by utilizing photochemical processes and ROS production. PDT’s safety profile is good, as it targets light-exposed sensitized cells, reduces harm to healthy tissues, and does not have systemic toxicity, making it a desirable alternative for patients with treatment tolerance or comorbidities.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent is not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Utility of photodynamic therapy in dentistry: Current concepts. Dent J (Basel). 2020;8:43.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical applications of antimicrobial photodynamic therapy in dentistry. Front Microbiol. 2023;13:1020995.
- [CrossRef] [PubMed] [Google Scholar]
- How Finsen's light cured lupus vulgaris. Photodermatol Photoimmunol Photomed. 2005;21:118-24.
- [CrossRef] [PubMed] [Google Scholar]
- Photodynamic therapy (part 1: Applications in dentistry) Int J Laser Dent. 2013;3:7-13.
- [CrossRef] [Google Scholar]
- Uber die wirkung der photodynamischen (fluorescierenden) stoffe auf protozoen und enzyme. Dtsch Arch Klin Med. 1904;80:427-87.
- [Google Scholar]
- Photodynamic therapy-mechanisms, photosensitizers and combinations. Biomed Pharmacother. 2018;106:1098-107.
- [CrossRef] [PubMed] [Google Scholar]
- Antimicrobial photodynamic therapy for the treatment of oral infections: A systematic review. J Dent Sci. 2023;18:1453-66.
- [CrossRef] [PubMed] [Google Scholar]
- Photodynamic therapy as a promising method used in the treatment of oral diseases. Adv Clin Exp Med. 2016;25:799-807.
- [CrossRef] [PubMed] [Google Scholar]
- Cell death in photodynamic therapy: From oxidative stress to anti-tumor immunity. Biochim Biophys Acta Rev Cancer. 2019;1872:188308.
- [CrossRef] [PubMed] [Google Scholar]






