Translate this page into:
Role of salivary and plasma melatonin levels in oral squamous cell carcinoma
*Corresponding author: Shamita Tiwari, Department of Oral Surgery, Saraswati Dental College and Hospital, Lucknow, Uttar Pradesh, India. shamita.dubey@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Aurora JK, Tiwari S, Tandon P, Saxena M, Singh P, Mishra P. Role of salivary and plasma melatonin levels in oral squamous cell carcinoma. Asian J Oral Health Allied Sci. 2023;13:10. doi: 10.25259/AJOHAS_22_2023
Abstract
Objectives:
The present research aims to look into the variations in levels of plasma and saliva melatonin with the sleep-wake cycle in healthy individuals and its correlation with the levels in oral squamous cell carcinoma (OSCC) patients and to explore the possibility of the use of plasma and saliva, as reliable, minimally-invasive/ non-invasive, and biofluids for measuring the levels of melatonin as biomarkers for the diagnosis of OSCC.
Material and Methods:
This comparative case–control prospective study was conducted on histopathologically proven 10 patients suffering from OSCC (Group II) and 10 healthy individuals (Group I) belonging to the age group of 25–65 years. The antecubital fossa region was used to draw peripheral venous blood, which was then centrifuged, and the plasma was kept at −200C. Using the passive drool technique, approximately 5 mL of unstimulated saliva samples were obtained, and the samples were kept at −200C. Both the samples were assayed as early as possible.
Results:
The result obtained was that the salivary and plasma melatonin levels were much higher during evening hours than morning interval in the healthy group (Group I). A similar trend was shown in Group II, compared to patients with OSCC, healthy patients have much higher levels of melatonin in their saliva and plasma.
Conclusion:
Hence, we conclude that the evaluation of the salivary melatonin level of patients suffering from OSCC may be considered reliable as well as non-invasive methods in the early diagnosis of OSCC. Saliva may be considered more advantageous over plasma for being non-invasive, less technique sensitive procedure, and having good patient compliance.
Keywords
Melatonin tumor marker
Oral squamous cell carcinoma
Biomakers
INTRODUCTION
The current estimates of age-standardized incidence and mortality for oral squamous cell carcinoma (OSCC) are 2.9/100,000 and 1.4/100,000 for women and 6.6/100,000 and 3.1/100,000 for men, respectively. The overall incidence and death associated with OSCC are rising. According to recent studies, a significant portion of cancer cases in some regions of India are related to mouth cancer.[1]
OSCC is becoming more common, with an estimated standardized incidence and mortality of 6.6/100,000 and 3.1/100,000 in men and 2.9/100,000 and 1.4/100,000 in women.[2]
At 70 years of age, the central region of India had the highest incidence of oral cancer in both males and females (64.8% and 37.2%, respectively). The west and northeast regions recorded the next highest magnitude (58.4% at 60 years of age).[3]
The gold standard investigative procedure for detection of malignancy is histopathological examination. Since this procedure is invasive, it causes discomfort to the patient and exposes the operator to the risk of transmission of certain infections. Moreover, the procurement of a biopsy requires the attainment of certain levels of surgical skill. Due to a lack of facilities and awareness, a major drawback of this technique is the delay in detection. Hence, there is a need for a non-invasive procedure for the early detection of malignancy, avoidance of profession-related hazards, and minimizing discomfort to the patient.
Tumor markers can be a non-invasive solution to our diagnostic needs. Plasma melatonin levels have been considered by researchers as a biomarker to detect breast, prostate, and lung malignancies. N-acetyl-5-methoxy tryptamine, or melatonin, was first identified and isolated in 1958. The pineal gland, retina, human and murine bone marrow cells, platelets, gastrointestinal tract, skin, and lymphocytes are the main organs that synthesize it.[4] Till date, no study investigating the role of plasma and salivary melatonin levels in malignancies of the oral cavity could be found. Therefore, this study investigates the role of plasma and salivary melatonin levels in patients of OSCC pre- and post-operatively.
Melatonin is known as the “chemical expression of darkness” because pineal melatonin synthesis is associated with nighttime[5] [Figure 1].

- Synthesis of melatonin.
Reviews revealed a wide range of melatonin effects that opened up new and significant possibilities for melatonin measurement as a biomarker (a biomarker for the early detection of certain disorders as well as a biomarker for their follow-up) and its therapeutic uses.[6]
MATERIAL AND METHODS
The present prospective case–control study was conducted in the Department of Oral and Maxillofacial Surgery, Saraswati Dental College, Lucknow, after obtaining approval from the “Institutional Human Ethics Committee (IHEC) and Institutional Research and Development Committee.
Twenty patients belonging to the age group of 25–65 years were randomly selected and divided into two groups after obtaining informed consent.
Group I
Ten healthy patients without having a history of OSCC or any other premalignant conditions, having no history of any systemic disease or other immunocompromised condition, whose age and socioeconomic status matches the subjects of the study group (the American Society of Anesthesiologists I health status).
Group II
Histopathologically proven ten patients suffering from OSCC who did not receive any treatment for OSCC before reporting at our institution.
Pregnant and lactating females, patients suffering from acute periodontal conditions or using drugs that interfere with the sleep-wake cycle or hypothalamic-pituitary-adrenal axis (e.g.: alprazolam, antipsychotic drugs, and carbamazepine) or those taking regular vitamin supplements such as folates, Vitamin B6, zinc, and magnesium or those consuming alcoholic beverages or excess of tomatoes, olives, barley, rice, walnuts, and caffeine, were excluded from the study.
Blood and saliva collection
Samples of saliva and peripheral venous blood were taken from each patient while they were in a relaxed, stress-free state, between the hours of 7 AM and 10 AM and 7 PM and 10 PM, to assess the daily pattern (diurnal variation) of melatonin secretion.
The blood was centrifuged, and the plasma was stored at −20°C. If a longer sample preservation period was required, a temperature of −80°C was maintained, and the sample was analyzed.
The passive drool method was used to gather 5 mL of unstimulated saliva samples. The sample was preserved using the same technique as plasma.
Determination of blood contamination in the saliva samples
A common strip test for determining whether free hemoglobin is present in urine, the Hemastix Reagent test, was used to detect blood contamination in saliva samples.
Plasma and salivary estimations
Melatonin concentration in plasma and saliva was measured using HUMAN MELATONIN (MT) ELISA KIT-EEL-H2016 [Figure 2].
- Melatonin kit along with reagents.
Statistical analysis
Chi-square test, unpaired t-test, and paired t-test were used to compare the outcomes among the various groups and analyze the data using descriptive statistics. Quantitative data were summarized as mean (standard deviation) and categorical data as proportions and percentages (%).
RESULTS
In Group I, the mean plasma level of melatonin during the morning time was lower than in the evening time, and the difference was statistically significant (P = 0.046) [Table 1 and Graph 1].
| Plasma | Melatonin | t-value | P-value | Interpretation | |
|---|---|---|---|---|---|
| Mean | SD | ||||
| Morning | |||||
| Group I | 103.27 | 39.94 | 6.51 | 0.001 | Vey highly significant |
| Group II | 20.42 | 4.84 | |||
| Evening | |||||
| Group I | 122.43 | 38.56 | 8.31 | 0.001 | Very highly significant |
| Group II | 20.03 | 5.55 | |||
SD: Standard Deviation; As P-value 0.001 is less than 0.05 which is statistically significant hence it is in bold.
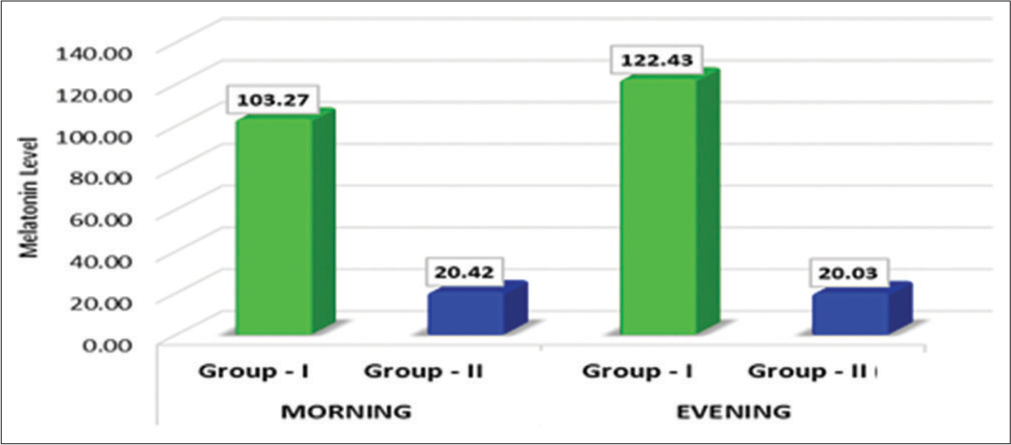
- Comparison of mean difference in plasma melatonin level between the groups.
Further, the mean salivary melatonin level during the morning was lower than in the evening time IN Group II, despite the fact that the difference was not found to be statistically significant (P = 0.055) [Table 2 and Graph 2] [Table 3 and Graph 3].
| Saliva | Melatonin | t-value | P-value | Interpretation | |
|---|---|---|---|---|---|
| Mean | SD | ||||
| Morning | |||||
| Group I | 138.79 | 70.46 | 3.08 | 0.006 | Highly significant |
| Group II | 58.81 | 42.06 | |||
| Evening | |||||
| Group I | 158.92 | 54.61 | 1.70 | 0.107 | Non-Significant |
| Group II | 100.45 | 94.39 | |||
SD: Standard Deviation; As P-value 0.006 is less than 0.05 which is statistically significant hence it is in bold.
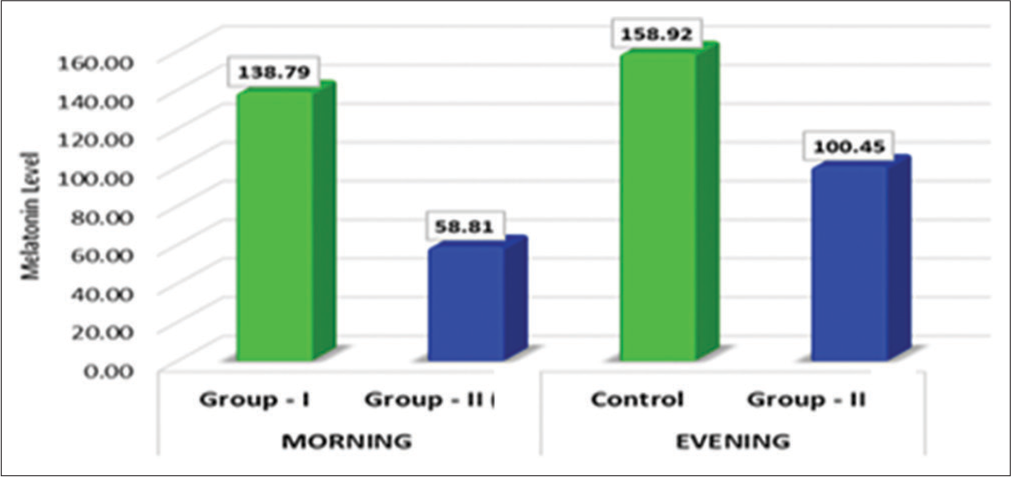
- Comparison of mean differences in salivary melatonin levels between the groups at morning and evening hours.
| Group-I | Melatonin | t-value | P-value | Interpretation | |
|---|---|---|---|---|---|
| Mean | SD | ||||
| Plasma levels | |||||
| Morning | 103.27 | 39.94 | 2.31 | 0.046 | Significant |
| Evening | 122.43 | 38.56 | |||
| Salivary levels | |||||
| Morning | 138.79 | 70.46 | 2.21 | 0.055 | Non-significant |
| Evening | 158.92 | 54.61 | |||
SD: Standard Deviation; As P-value 0.046 is less than 0.05 which is statistically significant hence it is in bold.

- Comparison of mean plasma and salivary melatonin levels during morning and evening in Group I.
DISCUSSION
In terms of both its composition and origins, saliva is a special and complicated oral fluid. It includes secretions from the nasal and pharyngeal mucosa, gingival crevicular fluid, oral mucosa transudate, non-adherent bacteria, desquamated oral epithelial cells, keratin debris, blood cells, and possibly leftover food or medication. It is composed of the secretions from the three major salivary glands (parotid, submandibular, and sublingual) as well as the minor glands.[7] Saliva’s many chemical components – water, inorganic compounds (ions), organic compounds (non-proteins and lipids), protein/polypeptides, and hormones – achieve key roles in processes such as lubrication, digestion, antimicrobial activity, aiding in the remineralization of tooth enamel, and maintaining normal taste perception.
α-amylase, albumin, cystatins, histatins, secretory- Immunoglobulin A, lactoferrin, mucins, lysozymes, proline-rich proteins, statherin, and transferrin are the most prevalent proteins in saliva, making up over 98% of the total salivary proteins. Salivary proteins also comprise the majority of potential OSCC salivary biomarkers.[7]
Plasma melatonin levels have been considered by researchers as a biomarker to detect the breast, prostate, and lung malignancies, but we could not come across any study investigating the role of plasma and salivary melatonin levels in malignancy of oral cavity. As a result, the present study attempted to look into the function of saliva and plasma melatonin levels as a biomarker for OSCC.
Age-related variations in blood melatonin peak levels occur in different people. Melatonin plasma levels in young, high secretors range from 54 pg/mL to 75 pg/mL, while levels in low- and elderly secretors range from 18 to 40 pg/mL.[8]
When comparing women with estrogen receptor-positive breast cancer to those with estrogen-negative disease or healthy matched control subjects, the amplitude of the nighttime peak of nocturnal plasma melatonin was smaller. This suggests that low levels of melatonin during the night may raise the risk of hormone-dependent breast cancer.[9] The observations made by the present study correlate with the results of the studies by Reiter.[4] Both studies show an increasing trend of mean plasma melatonin levels with time in healthy individuals (Group I) as well as in patients suffering from OSCC (Group II). The mean plasma and salivary levels of melatonin were found to be higher in healthy individuals than in OSCC patients.
Studies have demonstrated that patients with specific cancers have lower melatonin levels than healthy, normal individuals of the same age.[7] Our study depicted the same results, with mean plasma melatonin being highest in healthy individuals in Group I followed by Group II.
Vician et al. measured the levels of melatonin in the plasma of patients with colorectal carcinoma both before and after surgery, as well as in the plasma of patients with and without metastases of the cancer.[10] They noticed a rise in melatonin levels during the day and at night. According to Reiter’s study, patients who had liver metastases had more noticeable increases in melatonin levels.[4] Elevated levels of plasma melatonin could stem from either an upsurge in hormone production by the pineal gland in reaction to the strain of a major abdominal surgery, or an increase in melatonin production within the gut and its subsequent release into the bloodstream. In both situations, the higher melatonin levels following surgery might be a defense mechanism meant to boost immunity or shield the body from the damaging effects of free radicals.
Saliva contains melatonin, which may have significant effects on dental conditions, particularly periodontal disease. It is well-recognized that free radicals and changes in the immune system’s reaction to plaque-forming microbes can exacerbate periodontal diseases. According to the severity of periodontal disease, different levels of salivary melatonin were found in the recent research. Salivary melatonin levels decreased with increasing periodontal disease severity, suggesting that melatonin may function as a defense against external bacterial insults. Patients who have lower-than-normal salivary melatonin levels at admission, as can occasionally happen, particularly in the elderly or as a consequence of diseases involving malfunctioning salivary glands, may be more susceptible to oral cavity diseases.[11] The present study is consistent with these studies because it found that healthy subjects (Group I) had mean salivary melatonin levels higher than those of Group II.
The sensitivity and accuracy of the salivary melatonin assay were found to be similar to those of the gas chromatography/mass spectrometry assay of plasma melatonin, as reported by Voultsios et al. The time of salivary melatonin onset was found to have low intraindividual variability, and there was a significant correlation between salivary onset and plasma onset, according to these investigators. Likewise, there was a significant correlation observed between the acrophase (the moment when the rhythm peaks) of the saliva and plasma melatonin rhythms.[12] The observations made by the present study coincide with the results of this study, with saliva as well as plasma showing an increase in trend with an increase in time in Group I thus, showing linear correlation between them.
Merit and demerit of the study
Hence, the evaluation of salivary melatonin levels of patients suffering from OSCC may be considered reliable as well as non-invasive method in diagnosing the patient preoperatively. Furthermore, saliva may be considered more advantageous over plasma for being a non-invasive, less technique sensitive procedure, and safer and easily storable biofluid, along with good patient compliance. However, the same study needs to be conducted on a larger sample as well to fully establish the role of melatonin as a biomarker in OSCC patients.
CONCLUSION
The present study suggests that melatonin may be a reliable biomarker in diagnosing, preventing,and managing OSCC at the early stages. The findings of this study provide a solid foundation for future investigations into melatonin as an OSCC biomarker.
Ethical approval
The research/study approved by the Institutional Review Board at SARASWATI DENTAL COLLEGE AND HOSPITAL, number SDC/IHEC/2017/MDS/11, dated 18.1.2018.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Oral squamous cell carcinoma: Etiology, pathogenesis and prognostic value of genomic alterations. Indian J Cancer. 2006;43:60-6.
- [CrossRef] [PubMed] [Google Scholar]
- Oral cancer statistics in India on the basis of first report of 29 population-based cancer registries. J Oral Maxillofac Pathol JOMFP. 2018;22:18-26.
- [CrossRef] [PubMed] [Google Scholar]
- Cancer biomarkers: Are we ready for the prime time? Cancers. 2010;2:190-208.
- [CrossRef] [PubMed] [Google Scholar]
- Melatonin: The chemical expression of darkness. Mol Cell Endocrinol. 1991;79:C153-8.
- [CrossRef] [PubMed] [Google Scholar]
- Melatonin: Pharmacology, functions and therapeutic benefits. Curr Neuropharmacol. 2017;15:434-43.
- [CrossRef] [PubMed] [Google Scholar]
- Potential applications of human saliva as diagnostic fluid. Acta Otorhinolaryngol Ital. 2011;31:347-57.
- [Google Scholar]
- Saliva specimen: A new laboratory tool for diagnostic and basic investigation. Clin Chim Acta. 2007;383:30-40.
- [CrossRef] [PubMed] [Google Scholar]
- Melatonin in cancer management: Progress and promise. Cancer Res. 2006;66:9789-93.
- [CrossRef] [PubMed] [Google Scholar]
- Decreased nocturnal plasma melatonin peak in patients with estrogen receptor positive breast cancer. Science. 1982;216:1003-5.
- [CrossRef] [PubMed] [Google Scholar]
- Melatonin content in plasma and large intestine of patients with colorectal carcinoma before and after surgery. J Pineal Res. 1999;27:164-9.
- [CrossRef] [PubMed] [Google Scholar]
- Melatonin: Potential functions in the oral cavity. J Periodontol. 2007;78:1094-102.
- [CrossRef] [PubMed] [Google Scholar]
- Salivary melatonin as a circadian phase marker: Validation and comparison to plasma melatonin. J Biol Rhythms. 1997;12:457-66.
- [CrossRef] [PubMed] [Google Scholar]






