Translate this page into:
Non-surgical management of a large facial antibioma with photobiomodulation as a therapeutic adjunct: A case report
*Corresponding author: Rhythm Bains, Department of Conservative Dentistry and Endodontics, King George’s Medical University, Lucknow, Uttar Pradesh, India. docrhythm77@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Bains R, Bains VK, Verma P. Non-surgical management of a large facial antibioma with photobiomodulation as a therapeutic adjunct: A case report. Asian J Oral Health Allied Sci. 2023; 13:6. doi: 10.25259/AJOHAS_12_2023
Abstract
The injudicious overuse of antibiotics has led to a worldwide rise in antibiotic-resistant bacterial strains. Apart from antibiotic resistance and misuse of resources, inappropriate use of antibiotics also increases the possibility of potentially fatal anaphylactic reactions and makes patients vulnerable to unwarranted side effects. Sometimes, prolonged and unnecessary use of these antibiotics, either by the treating dentist or by the patient, combined with improper drainage of the pulpal canal, may lead to the formation of a tough fibrous swelling known as antibioma. The most commonly reported treatment modality for antibioma is surgical excision and extraction of the tooth involved. However, the present case reports a non-surgical management of a large facial antibioma using photobiomodulation (PBM) (neodymium-doped yttrium aluminum garnet, 1064 nm) for management of an antibioma developed in relation to a long-standing chronic apical periodontitis in mandibular molar.
Keywords
Antibioma
Antibiotics
Apical periodontitis
Low-level laser therapy
Nd:YAG
Nonsurgical management
Photobiomodulation
INTRODUCTION
The injudicious overuse of antibiotics has led to a worldwide rise in antibiotic-resistant bacterial strains. The fact that dental surgeons make approximately 10% of antibiotics prescription, and the prescription of antibiotics in dental clinics has increased from 6.7% to 11.3% in recent years has raised concerns over the contribution of dentists to the development of antibiotic-resistant bacteria.[1] Apart from antibiotic resistance and misuse of resources, inappropriate use of antibiotics also increases the possibility of potentially fatal anaphylactic reactions and makes patients vulnerable to unwarranted side effects.[2,3]
The primary mode of a necrosed or irreversibly damaged pulp is extirpation of infected pulp tissue along with adequate cleaning and shaping of the pulpal space, and cannot be managed with the use of antibiotics or anti-inflammatories alone. Sometimes, prolonged and unnecessary use of these antibiotics, either by the treating dentist or by the patient, combined with improper drainage of pulpal canal, may lead to a formation of a tough fibrous swelling known as antibioma. It presents as a localized tough fibrous swelling that is painless, smooth, non-tender, and firm on palpation.[4] In this condition, the abscess, instead of becoming pointing and draining, gets organized and fibrous. It presents with a dull pain and occasionally is associated with fever and systemic symptoms.[5] Literature reports, surgical removal as the most commonly used treatment for the management of antibioma, with only a few reporting the use of topical medicaments for resolving it non-surgically.[6]
In the past few years, treatment modalities have seen a paradigm shift from a more invasive to a more conservative or non-surgical/non-invasive approach. The use of biomaterials or techniques that induce a regenerative or repair cascade inside the body to heal itself is being sought.
Recently, the therapeutic role of laser light as an effective adjunct healing of dental conditions has gathered much interest, and positive healing outcomes have been documented for conditions such as post-extraction sockets, oral aphthous ulcers, post-surgery healing, and post-endodontic pain.[7] The term Photobiomodulation (PBM) is now accepted worldwide as the term for low-level laser therapy and is also referred to as photobiostimulation. It refers to the use of near-infrared lasers with a wavelength between 600 nm and 1000 nm with a power range from 5 mW to 500.[8] At present, it is practiced as part of physical therapy in many parts of the world and is used for musculoskeletal pain management, wound healing, and repair.[9] In recent years, authors have explored 1064 nm wavelength too, to be used for beneficial effects of PBM and have reported promising results.[10]
The present case reports describe the use of PBM with a flat top handpiece (neodymium-doped yttrium aluminum garnet [Nd:YAG], 1064 nm) wavelength for non-surgical management of a facial antibioma developed in relation to a long-standing chronic apical periodontitis in mandibular molar, and a prolonged and unsupervised usage of antibiotics by the patient.
CASE REPORT
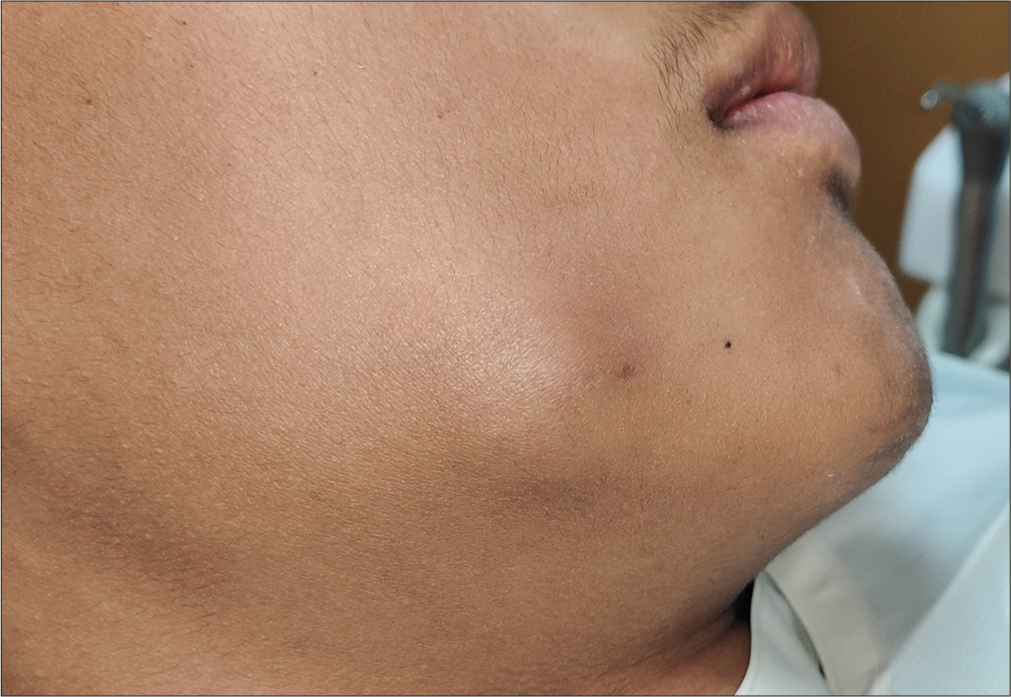
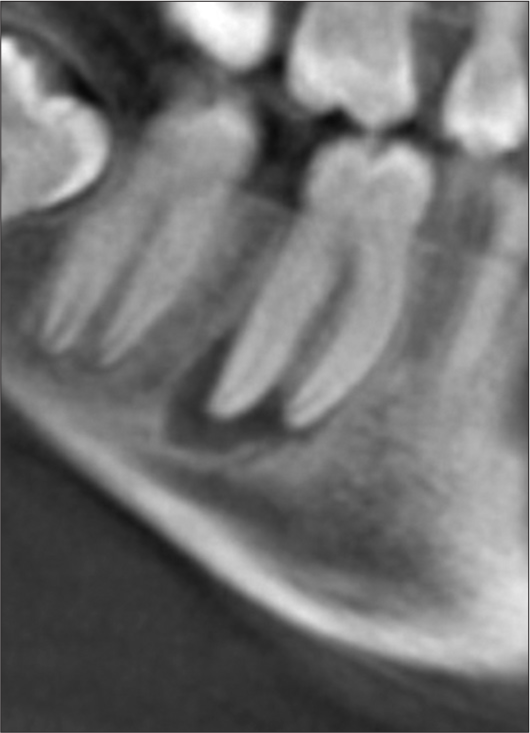
A 15-year-old, otherwise systemically healthy male patient reported to the endodontic clinic with the chief complaint of swelling on the right posterior region of the jaw since 15 days. Past dental history revealed that he had pain in the right mandibular first molar (46) for a month, accompanied by a diffuse swelling on the right side of the face. For this, he had taken some self-medication (Amoxicillin 500 mg, thrice daily for five days). When the swelling did not subside after five days, he visited a private clinic where he was prescribed another course of antibiotics for five days (Amoxicillinclavulanic acid 625 mg, thrice daily for 5 days), following which the swelling became smaller in size. At present, the swelling was localized, approximately 2 cm × 2 cm in size, and hard on palpation [Figure 1]. Intraoral examination revealed grossly carious 46, with vestibular obliteration in relation to it. The tooth was not tender to percussion. Pulp sensibility testing with an electronic pulp tester recorded no response. The pre-operative cone-beam computed tomography image revealed a carious pulpal exposure and a periapical lesion (Ostarvik’s PAI score 4) on the distal root of 46 [Figure 2]. A diagnosis of asymptomatic apical periodontitis with pulpal necrosis was made for the tooth, and based on the history given by the patient and its appearance, a diagnosis of antibioma was made for the extraoral swelling.
- Pre-operative computed tomography scan of tooth number 46 revealing a per-apical lesion.
- Pre-operative clinical view showing facial antibioma.
A treatment plan of root canal treatment for 46 and noninvasive management of antibioma with laser PBM was decided, and a written informed consent was taken from the guardian of the patient. Keeping in mind the current COVID-19 scenario, the patient was told to pre-rinse with 0.2% betadine for 30 seconds, and a rubber dam was applied for isolation. The soft caries were removed as much as possible with the help of a sharp spoon excavator, and rest access to the pulp space was gained with the help of a micromotor. The canals were prepared up to size F2 and copiously irrigated with normal saline and 5.25% sodium hypochlorite. A non-setting calcium hydroxide paste was placed in the canals as an intracanal medicament. Thereafter, the apical area in relation to 46 was irradiated for PBM with Fotona R24 flat-top handpiece (Fotona AT Fidelis) 0.5 W, 10 Hz in MSP modality, 60 s/1 cm2. The PBM therapy was given on day 1, 3, 5, and 7 [Figure 3]. After seven days, the canals were obturated with corresponding gutta-percha points, and AH-plus sealer, and access cavity were restored with a posterior composite resin. After 15 days, the swelling had reduced [Figure 4]. Thereafter, the patient was recalled every week for a month and was evaluated for the size of the swelling, radiographic healing of the periapical lesion, or any new signs or symptoms. When last recalled six months after the treatment, the patient was asymptomatic, without any fresh complaints and exhibited adequate radiographic healing (PAI score 1) [Figure 5].
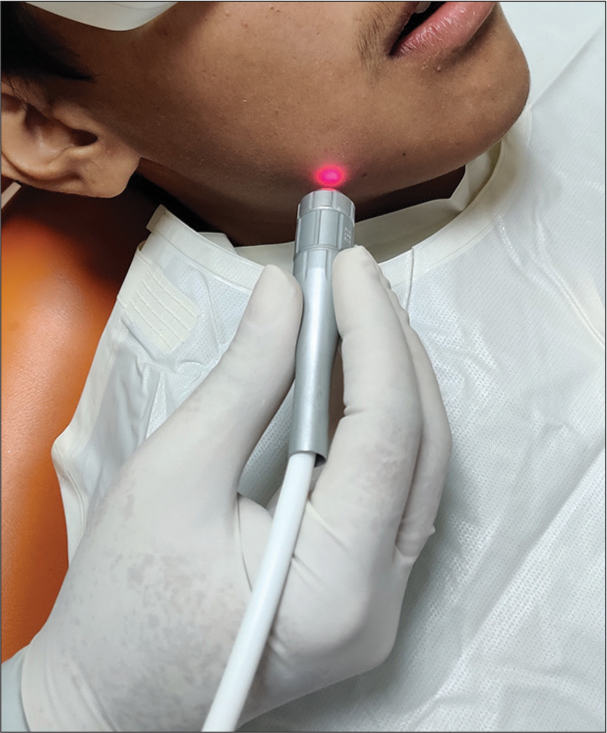
- Laser photobiomodulation of antibioma with flat-top R-24 handpiece.
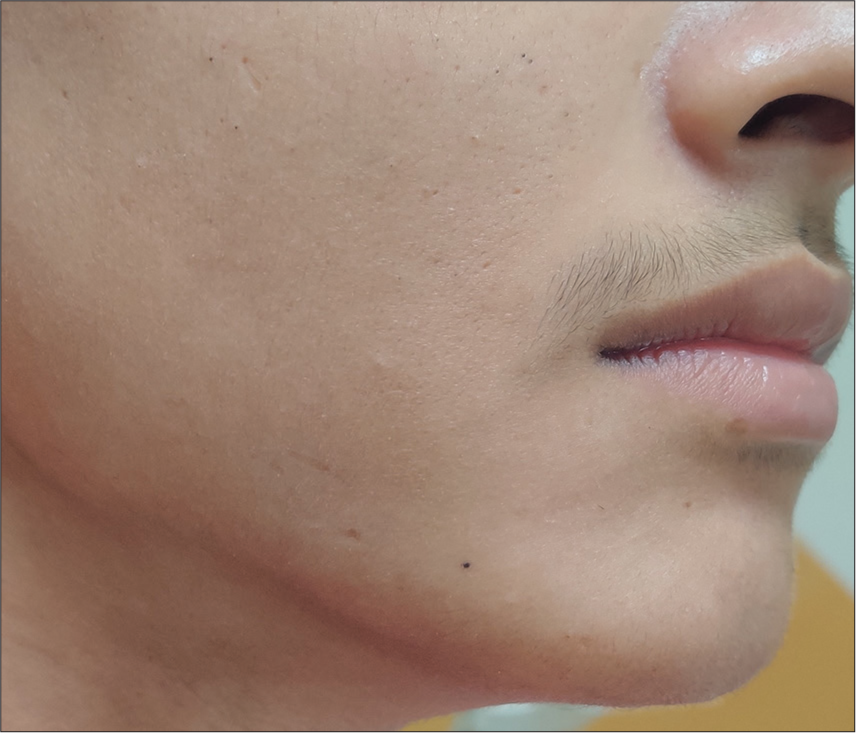
- Post-operative clinical view.

- Six months post-operative follow-up intraoral periapical (IOPA) X-ray.
DISCUSSION
Antibioma is often the result of prolonged and unnecessary use of antibiotics, inadequate cleaning and disinfection of the pulp space, and a lack of post-operative follow-up. Clinically, it presents as a painless, smooth, and non-tender swelling that feels hard on palpation.[11] In the present case too, the patient was undergoing root canal therapy for the right mandibular first molar, but the treatment was not complete, and the patient was then on 15 days of antibiotics, leading to the present condition of the patient.
Apart from the novel use of Nd:YAG PBM for non-invasive management of the condition, the present paper highlighted two other major concerns. First is the negligence on the part of the patient that swelling of this nature is a sequel to endodontic infection and can be resolved with non-surgical endodontic treatment; and second, the indiscriminate use of antibiotics both by the patient and the clinician. Misuse and overuse of antibiotics cause bacteria to adapt to the antibiotics that are prescribed to eradicate them. According to the Center for Disease Control, there are numerous lethal bacteria against which only limited antibiotics work, making treatment of infections associated with these pathogens more expensive and less efficacious.[12,13]
The most widely reported and commonly practiced method for management of antibioma is the incision and drainage of the swelling, or extraction of the diseased tooth.[5,11] However, few case reports have highlighted a non-surgical approach as its treatment approach. In an interesting case, a regular dressing of magnesium sulfate (Epsom salt) and glycerin was applied over the swelling that worked by bringing up the infected pus to the surface of the skin, and consequently bursting it, thereby reducing the swelling.[2] One another case report described the use of intralesional injections of Triamcinolone acetonide 10 mg/mL in combination with broad-spectrum b-lactam antibiotic (Inj. Amoxiclav 1000/200 mg) to bring about resolution of the lesion.[6]
In the present case, a non-surgical treatment approach was undertaken with the help of laser PBM is the therapeutic use of light (low power laser or LED; range: 1–500 mW) to a pathological condition with the aim to stimulate tissue regeneration, diminish inflammation, and alleviate pain.[14] The spectrum of this therapeutic light, also called the therapeutic window, is within the red or near-infrared (NIR) spectrum (600–1000 nm), with a power density (irradiance) between 1 MW and 5 W/cm2. The mechanism of action of PBM is not ablative or thermal, but photochemical, analogous to photosynthesis in plants in which the light is absorbed and induces a chemical change (dose-response).[15]
PBM mainly depends on the interaction of light with tissue biology. This effect is highest when light penetration is the deepest and photo-acceptor absorption is the maximum. In mammalian tissues, the main chromophores that absorb light in this NIR spectroscopy range are hemoglobin, myoglobin, melanin, and mitochondrial Cytochrome c Oxidase.[16] Many theories have been proposed regarding the mechanism of action of PBM. It is believed that PBM increases the rate of electron transfer by the respiratory chain, thus hastening ATP production. Second, it was hypothesized that it stimulates the production of nitrous oxide, which could further enhance wound healing by vasodilatation and indirectly regulate transcription over many mammalian genes. In addition, PBM could have antagonist effects on reactive oxygen species formation, which again helps in wound healing and repair.[17]
In the present case, the use of Nd:YAG 1064 nm was made for PBM. The literature does not say much about the use of the Nd:YAG in PBM. Majority of the research in this field has been conducted regarding wavelengths in the range from 400 nm to 980 nm as in this range of wavelengths. Nd:YAG, at a wavelength of 1064 nm, is near this window and has an advantage of having a reasonably effective penetration up to the deeper tissues.[18]
Recently, in a study by Usumez et al.,[19] it was seen that low-level Nd:YAG laser therapy hastens the wound healing process by changing the expression of platelet-derived growth factor (PDGF) and basic fibroblast growth factor (bFGF) genes responsible for the stimulation of the cell proliferation and fibroblast growth. Furthermore, they stated that no statistically significant differences were seen when compared to among the groups using 660 nm, 810 nm, and 980 nm wavelengths.
The Arnd Shultz Law,[20] which states that “For every substance, small doses stimulate, moderate doses inhibit, large doses kill” is applicable to the way PBM interacts with tissues. It is of paramount importance to elucidate energy dosage and parameters that will effectively promote positive change in individual cells while avoiding negative effects. Karu observed that high fluences cause the destruction of photoreceptors, which is accompanied by growth inhibition and cell lethality.[21] The World Association of Laser Therapy advises to apply energy in the range from 3 J/cm2 to 10 J/cm2 to obtain effective biostimulation and also avoid bioinhibitory effects.[22] A scanning or stepping motion is recommended rather than application over a single spot due to the Gaussian beam profile of clinical lasers.[23] If applied on a single spot, cells directly in the center of the beam will be irradiated at a very high fluence, compared to those at the outer margins. As a result, there will be chances of cells at the beam center to get exposed to far above the advocated range of 3–10 J/cm2. This causes bioinhibition at the center due to high dose, while the peripheral cells receive insufficient cellular energy to produce any beneficial effect.
CONCLUSION
The present case report highlights promising results regarding the role of PBM as a non-invasive and non-surgical adjunct to endodontic treatment in the healing of antibioma lesion. Furthermore, it calls for a need for awareness among the public and education among oral health-care providers regarding the judicious prescription of antibiotics.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Antibiotic prescribing by dentists has increased: Why? J Am Dent Assoc. 2016;147:320-7.
- [CrossRef] [PubMed] [Google Scholar]
- Role of antibiotics in orofacial antibioma and its management: A case report. J Sci Dent. 2019;9:13-4.
- [CrossRef] [Google Scholar]
- Antibiotic use in dental practice. A review. Med Oral Patol Oral Cir Bucal. 2007;12:E186-92.
- [Google Scholar]
- Use of antibiotics in pediatric dentistry: Not a child's play. Int J Periodontol Implantol. 2017;2:109-11.
- [Google Scholar]
- Large antibioma resulting from injudicious use of antibiotics: A case report. Int J Dent Med Res. 2015;1:89-92.
- [Google Scholar]
- Non-surgical treatment of antibioma in oro-facial region. Int J Curr Res. 2017;9:56666-7.
- [Google Scholar]
- Photobiomodulation and oral wound healing. Indian J Multidiscip Dent. 2017;7:129-34.
- [CrossRef] [Google Scholar]
- Photobiomodulation or low-level laser therapy. J Biophotonics. 2016;9:1122-4.
- [CrossRef] [PubMed] [Google Scholar]
- Photobiomodulation therapy for wound care: A potent, noninvasive, photoceutical approach. Adv Skin Wound Care. 2019;32:157-67.
- [CrossRef] [PubMed] [Google Scholar]
- Case studies on the use of a new flat-top handpiece for biomodulation in dentistry and medicine. J Laser Health Academy 2015:1-6.
- [Google Scholar]
- Antibiotic resistance threats in the United States. 2013. United States: Centers for Disease Control and Prevention; Available from: http://www.cdc.gov/drugresistance/pdf/arthreats-2013-508.pdf [Last accessed on 2023 July 3]
- [Google Scholar]
- Considerations for responsible antibiotic use in dentistry. J Am Dent Assoc. 2016;147:683-6.
- [CrossRef] [PubMed] [Google Scholar]
- Low-level light/laser therapy versus photobiomodulation therapy. Photomed Laser Surg. 2015;33:183-4.
- [CrossRef] [PubMed] [Google Scholar]
- Craniofacial wound healing with photobiomodulation therapy: New insights and current challenges. J Dent Res. 2016;95:977-84.
- [CrossRef] [PubMed] [Google Scholar]
- Photobiomodulation in oral medicine: A review. J Investig Clin Dent. 2016;7:114-26.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanisms of photobiomodulation (PBM) focused on oral mucositis prevention and treatment: A scoping review. BMC Oral Health. 2021;21:220.
- [CrossRef] [PubMed] [Google Scholar]
- Electromagnetic radiation In: Cameron MH, ed. Physical agents in rehabilitation: From research to practice (3rd ed). Philadelphia, PA: Saunders; 2009. p. :303-44.
- [Google Scholar]
- Effects of laser irradiation at different wavelengths (660, 810, 980, and 1,064 nm) on mucositis in an animal model of wound healing. Lasers Med Sci. 2014;29:1807-13.
- [CrossRef] [PubMed] [Google Scholar]
- Biphasic dose response in low level light therapy. Dose Response. 2009;7:358-83.
- [CrossRef] [PubMed] [Google Scholar]
- Low-power laser effects In: Waynant RW, ed. Lasers in medicine. Boca Raton: CRC; 2002. p. :171-210.
- [CrossRef] [Google Scholar]
- Recommended treatment doses for low level laser therapy. Available from: https://www.walt.nu [Last accessed on 2021 Jul 15]
- [Google Scholar]
- Efficacy of low-level laser therapy (LLLT) in oral mucositis: What have we learned from randomized studies and meta-analyses? Photomed Laser Surg. 2012;30:191-2.
- [CrossRef] [PubMed] [Google Scholar]






